Distillation Tutorial VII: Energy Considerations for Multi-Unit Processes (Main Page)
Distillation arises out of a need to separate chemical species for different uses &mdash whether it is the separation of a crude oil into fractions for the blending and subsequent production of various fuels or the separation of raw materials, by-products and products in a typical reaction/separation/recycle process. Thus, a distillation column is often one in several unit operations within a process in a chemical or petroleum plant. While the total energy consumed in multi-unit processes is frequently dominated by distillation, it is important to be able to understand and analyze energy consumption in multi-unit processes. Common examples of multi-unit processes include (1) hybrid separation processes like extraction-distillation, (2) reaction/separation/recycle processes, and (3) distillation trains (or sequences).
Tutorials I-VI focused on various aspects of the synthesis and design of single distillation columns for mixtures with two or more components. In this tutorial, the student will learn
- The challenges presented by processes with multiple units in which distillation is a part.
- Basic concepts of liquid-liquid phase equilibrium.
- To aply the shortest stripping line distance approach in a multi-unit process.
- Basic concepts of the equation-tearing approach to process flowsheet computations.
Motivation for the Use of Hybrid Separation Processes. The motivation for hybrid separations comes from the well known fact that some distillation processes are extremely energy intensive. A good example of this fact is the separation of dilute acid solutions by distillation. The reason straight distillation is energy intensive is because building block acids like acetic acid and formic acid have higher boiling points that water. As a result, the acids generally are removed as the bottoms product of any distillation and water is taken overhead as the distillate. For dilute acids this means that significant amounts of water are vaporized in the stripping section of the column and subsequently condensed as reflux in the rectifying section of the column. This boiling and subsequent condensing of water is very wasteful from an energy perspective since water has a fairly high heat of vaporization.
Conventional Distillation of Acid-Water Mixtures. To understand the energy consumption of dilute acid-water distillation, consider the distillation of 10,000 lb/h (438.89 lbmol/h) of a saturated liquid containing 30 wt % (11.5 mol %) acetic acid in water at atmospheric pressure. Let the bottoms and distillate compositions be xB = (0.9999, 0.0001) and xD = (0.001, 0.999) respectively, where the components are in the order acetic acid (1) and water (2). In the calculations that follow, we use the UNIQUAC equation to model the liquid phase and the Hayden-O'Connell equation for the vapor phase since acetic acid forms dimers.
Design Calculations: Using the shortest stripping line approach (or the McCabe-Thiele method), we find a feed pinch in the stripping section of the column at a boil-up ratio of 20.97; thus smin = 20.97. Using the feed, bottoms and distillate compositions, we calculate a corresponding minimum reflux ratio of rmin = 1.701. The corresponding distillate and bottoms flow rates are D = 388.80 lbmol/h and B = 50.09 lbmol/h. There are 300 stripping stages and 165 rectifying stages in this pinched, minimum energy design.
Energy Consumption: Using latent heats of vaporization of λAA = 10,431.60 Btu/lbmol and λW = 17,465.22 Btu/lbmol and the approximate relationship that QR ≈ VλAA = sminBλAA (since the bottoms is essentially all acetic acid), we find that QR = 10.957 MBtu/h.
Exercise Using the same feed composition, feed flow rate, and distillate composition from the illustration above, show that changing the bottoms composition to xB = (0.995, 0.005) does little to effect the energy consumption of acetic acid-water distillation.
Hybrid Separation Processes. One alternative to the binary distillation of dilute acid solutions is to use a hybrid separation process like the one shown in Figure 1.

Figure 7.1: Hybrid Separation Process for Acetic Acid
While there are many hybrid separation processes (e.g., extraction-distillation, reactive distillation, distillation-pervaporation, membrane separation-distillation, and so on), extraction-distillation is suitable for acid-water separation. In extraction-distillation, a solvent is used to first extract the acid from the water. This extraction is followed by a primary distillation of the acid-solvent mixture (which often contains only a small amount of water) to recover the acid and then a second distillation to recover the solvent, since solvent is generally expensive.
This extraction-distillation approach has the following advantages:
- It removes the need to 'boil' large amounts of water, thereby reducing energy demands by reducing throughput.
- The choice of solvent can further reduce energy demands if the solvent is significantly more volatile than the acid.
However, the use of extraction-distillation is not without drawbacks.
- It is a much more complicated process than binary distillation.
- Recycle is necessary in order for the extraction-distillation process to be profitable and these recycle streams can often create other challenges with regard to process operation and control.
- Organic solvents are generally not environmentally friendly and often hazardous. Thus they are typically regulated chemicals.
Process Synthesis and Design of Extraction-Distillation Processes. Again we choose the separation of 10,000 lb/h of a 30 wt % (11.5 mol %) solution of acetic acid in water as an illustrative example. The desired product from this extraction-distillation process is a 99.99 mol % acetic acid stream from the bottom of the acid recovery column. There is also a need to
- Minimize the amount of make-up (fresh) solvent, since solvent is expensive and trace amounts will be lost in both the acid product and the wastewater stream shown in Fig. 1.
- Produce a wastewater stream reasonably devoid of contaminants.
- Operate the entire process with the least energy consumption possible.
Design Decision #1: The process synthesis for extraction-distillation begins with the structural decisions shown in Figure 1, in which we have placed the extraction unit first in order to reduce energy demands, followed by the acid recovery and solvent recovery columns respectively, and have required recycling for better process economics.
Design Decision #2: The structural decisions are followed by the choice of solvent, which in this case is ethyl acetate.
Design Decision #3:The next set of decisions that must be made, and which strongly impact the energy demands and economics of the extraction-distillation process, include
- How much separation should be performed in the extraction column?
- How much make-up solvent is required?
- How many stages are there in the acid recovery column?
- How many stages are there in the solvent recovery column?
- What is the minimum amount of energy that can be used to produce the desired product(s)?
Because it contains two recycle streams, all of the units in the extraction-distillation process are linked or coupled. Thus it is a non-trivial task to perform steady-state mass balance and phase equilibrium calculations for this process, which are required to answer the questions posed above (as well as others). Moreover, both vapor-liquid and liquid-liquid phase equilibria are involved (as indicated by the decanter).
How to proceed? At this point, the student may be wondering — where and how do I begin to perform the relevant calculations to get at the energy demands for this complicated process? Here is an overview of one way to analyze this process using what is called a sequential modular approach to process flowsheeting.
Step 1: Estimate the amount of make-up solvent needed. It is often reasonable to make an initial guess of zero for the flow of this stream.
Step 2: Perform 'tearing' of recycle streams in order to temporarily isolate the calculations for each unit. With reference to Figure 1, we 'break' the recycle stream from the decanter to the extractor and the recycle stream from the solvent recovery column to the decanter. However, when we do this, we need to 'guess' at the properties of the 'torn' streams because they are both inputs to one unit and outputs of another unit in the process. Guessing values for the variables in the 'torn' streams allows us to march from unit to unit, performing calculations in much the way the material flows through the process.
Step 3: After all calculations for each unit are performed, we need to check to see if the calculated values of the tear stream variables are the same as the guessed values of the tear stream variables. If they are, then we are done and all model equations for the process should be satisfied. If not, we must guess new values of the tear stream variables and repeat step 1.
Step 4: Repeat steps 1, 2 and 3 until the make-up flow rate and all values of the tear stream variables are the same as all guessed values of the make-up stream flow rate and tear stream variables (to some prescribed accuracy).
While this sequential modular approach to the synthesis and design of the extraction-distillation process might seem straightforward, it contains a large number of different types of computations as well as design decisions that must be uncovered. In the material that follows, we take the student through the calculations for this extraction-distillation process. We caution the student that the calculations that follow are quite detailed, lengthy, and highly inter-dependent. A mistake in any one of these calculations has a rippling effect throughout the remaining analysis. Remember, the devil is in the details.
Detailed Calculations for the Extraction-Distillation Process
Flowsheet Iteration #0: This set of calculations initializes
the process
flowsheet.
- Liquid-Liquid Extraction Column. Here we know the feed to the
extractor and assume the solvent is pure solvent. Knowledge of the
bimodal curve and the stripping pinch point curve for the primary acid
recovery column gives a composition for the extract stream of
xT = (0.0890, 0.6204, 0.2906). This information is sufficient
to calculate the flow of solvent, S = 354.310 lbmol/h, the extract flow,
E = 567.036 lbmol/h, and the flow rate and composition of the raffinate
stream, which is R = 226.167 lbmol/h and xR = (0.0000272,
0.01114, 0.98883), where the components are in the order acetic acid
(1), ethyl acetate (2), water (3).
Quick Exercise. How much acetic acid is extracted from the feed to the process? How much is lost in the raffinate stream? - Primary Acid Recovery Column. For the primary acid recovery
column, we know two important facts — the bottoms product
composition and the fact that the feed must lie on the liquid-liquid
equilibrium (binodal) curve. The bottoms composition is xB =
(0.9999, 0.00005, 0.00005). The feed to the column is the extract
composition xT = (0.0890, 0.6204, 0.2906) and the initial
estimate of the etract flow from the extractor is the feed flow to the
acid recovery column. From this information, we can easily calculate a
minimum boil-up ratio of smin = 10.92 using the shortest
stripping line distance approach and give yD = (0.0056,
0.6771, 0.31730, V = D = 519.474 lbmol/h and B = 47.562 lbmol/h. Note
that we are assuming that the stripper is feed pinched.
Quick Exercise. Calculate the amount of acetic acid recovered in the primary acid recovery column. - Decantation. Here we assume the recycle stream from the
solvent recovery column to the decanter is zero. Thus only the
distillate product from the primary acid column is initially separated
into an organic phase (S1) and a water phase (S2)
in a decanter. Table 1 gives the results for the decanter calculations,
which were performed using Gibbs free energy minimization
Table 1: Decanter Stream Variables for Hybrid Separation of Acetic Acid/Water Stream Flow Rate (lbmol/h) Composition S1 422.840 (0.00627, 0.82907, 0.16466) S2 96.634 (0.00265, 0.01199, 0.98626)
Quick Exercise. Perform mass balance calculations on the decanter.
- Solvent Recovery Column. The feed to the solvent recovery
column is the sum of the raffinate flow and the flow of the water stream
from the decanter, so F = 226.167 + 96.634 = 322.801 lbmol/h. The
corresponding composition of the feed to the solvent recovery column can
be calculated by component mass balance and gives a feed composition of
(0.000812, 0.01139, 0.98779). Here again we assume the column is a feed
pinched stripper so the distillate composition, yD, from
solvent stripper is in a phase equilibrium with the feed composition to
the solvent recovery column. This gives yD = (0.00115,
0.64206, 0.35782). To get and estimate of the vapor flow up the column
(or distillate product) we must assume a bottoms composition from the
solvent stripper. Here we assume xB = (0.0005, 0.0005,
0.9990). This gives V = D = 5.479 lbmol/h, B = 317.322 lbmol/h and a
boil-up ratio of s = 0.01726. Note that the energy demands for this
solvent stripper are insignificant. Also, note that since the bottoms
stream from this column is essentially water, we can use sparged steam
and not incur the cost of a reboiler for the solvent stripper.
Quick Exercise. At this point of the calculations, how much acetic acid is lost in the wastewater stream? Is the overall acetic acid mass balance for the process satisfied? If not, what is the error?
Flowsheet Iteration #1: This iteration begins the iteration
process for
the entire process flowsheet. The primary flowsheet iteration variables
are the solvent recycle flow and composition to the liquid-liquid
extraction column.
- Extraction Column. The initial estimate of the flowsheet
iteration variables are S1 + D (solvent recovery column) =
448.63
lbmol/h and xS1 = (0.00627, 0.82907, 0.16466). Remember, the
extract the
target extract composition is xT = (0.0890, 0.6204, 0.2906).
Liquid-liquid extraction mass and phase equilibrium calculations give
new estimates of the extract flow, raffinate flow rate and composition,
and the solvent make-up flow. These calculations give the following
results:
Table 2: Extract and Raffinate Stream Variables from Liquid-Liquid Extraction Column Stream Flow Rate (lbmol/h) Composition Extract 601.63 (0.0890, 0.6204, 0.2906) Raffinate 287.182 (0.0, 0.00292, 0.99708) - Primary Acid Recovery Column. Returning to the primary acid recovery column with the same bottoms composition specifications of xB = (0.9999, 0.00005, 0.00005) and minimum boil-up ratio of s = 10.92 gives B = 50.472 and D = 551.158 lbmol/h. The composition of the overhead product was yD = (0.0056, 0.6771, 0.3173). Here the flow rates and compositions have changed only slightly.
- Decanter. Here the recycle stream from the solvent recovery
column is considered and has the flow rate and composition from the
previous flowsheet iteration. As with the primary acid recovery column,
it is the flow rates of the product streams from the decanter that
change significantly. The compositions of the organic and water phases,
on the other hand, change very little. Table 4 gives the values of the
stream variables for the decanter.
Table 3: Decanter Stream Variables for Hybrid Separation of Acetic Acid/Water Stream Flow Rate (lbmol/h) Composition S1 448.63 (0.0063, 0.8291, 0.1646) S2 109.247 (0.00270, 0.0120, 0.9863) Exercise: Verify that streams S1 and S2 satisfy liquid-liquid equilibrium using the UNIQUAC equation. Also verify that the component mass balances for the decanter are satisfied.
- Solvent Recovery Column. The feed to the solvent recovery
column
has changed significantly since it is the sum of the raffinate flow and
the flow of the water stream from the decanter, so F = 287.182 + 109.247
= 395.117 lbmol/h. Compare this to the previous iteration where F =
322.801 lbmol/h. The corresponding composition of the feed to the
solvent recovery column is (0.0008, 0.01139, 0.98779). Here again we
assume the column is a feed pinched stripper so the distillate
composition, yD, from solvent stripper is in phase
equilibrium with the
feed composition to the solvent recovery column. This gives
yD =
(0.0012, 0.6421, 0.3578). Mass balance can be used to calculate the
bottoms composition from the solvent recovery column, xB =
(0.0005,
0.0033, 0.9962). This gives V = D = 6.719 lbmol/h, B = 395.117 lbmol/h
and a boil-up ratio of s = 0.0170. Note that the energy demands for this
solvent stripper remain insignificant.
Important Note: Once the flow and composition of the solvent recovery column overhead stream is considered, there is another level of iteration. Note that the presence of this stream affects the feed to the decanter, streams S1 and S2 and, in turn, the feed to the solvent recovery column. Thus we must iterate between the decanter and the solvent recovery column until convergence of this inner loop is achieved. Fortunately the magnitude of the overhead stream is small so it has a somewhat small effect on convergence suggesting that inner loop iteration could possibly be neglected.
Flowsheet Iteration #2: The remaining iterations
for process flowsheet are essentially the same as flowsheet
iteration #1. The key concept here is that these iterations
adjust stream flows but not stream compositions, which
remain roughly the same from iteration to iteration. At the
flowsheet level, the primary variables are the solvent
recycle flow and composition to the liquid-liquid extraction
column, from which the make-up solvent flow can be
calculated.
- Extraction Column. The initial estimate of the
flowsheet iteration variables are S1 (solvent from decanter)
=
448.63 lbmol/h and xS1 = (0.00627, 0.82907, 0.16466).
Remember,
the target extract composition is xT = (0.0890, 0.6204,
0.2906).
Liquid-liquid extraction mass and phase equilibrium calculations
give new estimates of the extract flow, raffinate flow rate and
composition, and the solvent make-up flow. These calculations
give the following results:
Table 4: Extract and Raffinate Stream Variables from Liquid-Liquid Extraction Column Stream Flow Rate (lbmol/h) Composition Extract 592.896 (0.0890, 0.6204, 0.2906) Raffinate 294.625 (0.0027, 0.0111, 0.9862) On this iteration, the stream flows and compositions for the extract and raffinate flow rates have changed very little. The calculated value of the solvent make-up stream stayed constant at 1.292 lbmol/h.
- Primary Acid Recovery Column. Returning to the
primary
acid recovery column with the same bottoms composition specifications of
xB = (0.9999, 0.00005, 0.00005) and minimum boil-up ratio of
s = 10.92
gives B = 49.740 lbmol/h and D = 543.156 lbmol/h. The composition
of the overhead product was yD = (0.0056, 0.6771,
0.3173). Here the flow rates and compositions have changed only
slightly.
- Decanter Here the recycle stream from the solvent
recovery column is considered and has the flow rate and composition
from the previous flowsheet iteration. As with the primary acid
recovery column, it is the flow rates of the product streams from
the decanter that change significantly. The compositions of the
organic and water phases, on the other hand, change very little.
Table 5 gives the values of the stream variables for the decanter.
Table 5: Decanter Stream Variables for Hybrid Separation of Acetic Acid/Water Stream Flow Rate (lbmol/h) Composition S1 447.325 (0.00616, 0.8293, 0.1645) S2 102.557 (0.00260, 0.0111, 0.98631) Exercise: Verify that streams S1 and S2 satisfy liquid-liquid equilibrium using the UNIQUAC equation. Also verify that the component mass balances for the decanter are satisfied.
- Solvent Recovery Column. The feed to the solvent recovery column has changed significantly since it is the sum of the raffinate flow and the flow of the water stream from the decanter, so F = 294.625 + 102.557 = 397.182 lbmol/h. The corresponding composition of the feed to the solvent recovery column has changed very little from (0.0008, 0.01139, 0.98779) to (0.00267, 0.01110, 0.98623). Here again we assume the column is a feed pinched stripper so the distillate composition, yD, from solvent stripper is in phase equilibrium with the feed composition to the solvent recovery column. This gives yD = (0.00402, 0.62821, 0.37138). Mass balance can be used to calculate the bottoms composition from the solvent recovery column, xB = (0.0018, 0.0032, 0.9949). This gives V = D = 4.963 lbmol/h, B = 396.848 lbmol/h and a boil-up ratio of s = 0.0125.
Flowsheet Iteration #3: The primary flowsheet iteration
variables are the solvent recycle flow and composition to the
liquid-liquid extraction column.
- Extraction Column. The initial estimate of the flowsheet
iteration variables are S1 = 447.325 lbmol/h and
xS1 = (0.00616, 0.8293,
0.1645). Again, the target extract composition is xT =
(0.0890, 0.6204,
0.2906). Liquid-liquid extraction mass and phase equilibrium
calculations give new estimates of the extract flow, raffinate flow rate
and composition, and the solvent make-up flow. These calculations give
the following results:
Table 6: Extract and Raffinate Stream Variables from Liquid-Liquid Extraction Column Stream Flow Rate (lbmol/h) Composition Extract 591.608 (0.0890, 0.6204, 0.2906) Raffinate 294.607 (0.0026, 0.0111, 0.9863) The solvent make-up flow is again 1.292 lbmol/h. Note that the extract and raffinate flow rates have changed significantly but that the compositions of these streams changed very little.
- Primary Acid Recovery Column. Returning to the
primary
acid recovery column with the same bottoms composition specifications of
xB = (0.9999, 0.00005, 0.00005) and minimum boil-up ratio of
s = 10.92
gives B = 49.632 lbmol/h and D = 541.976 lbmol/h.
- Decanter. Again, the feed to the decanter now consists
of the overhead from both columns and iteration is recommended. As with
the primary acid recovery column, it is the flow rates of the product
streams from the decanter that change significantly. The compositions of
the organic and water phases, on the other hand, change very little.
Table 7 gives the values of the stream variables for the decanter.
Table 7: Decanter Stream Variables for Hybrid Separation of Acetic Acid/Water Stream Flow Rate (lbmol/h) Composition S1 445.401 (0.00661, 0.82828, 0.16511) S2 101.556 (0.0028, 0.0113, 0.9861) - Solvent Recovery Column. The feed to the solvent recovery column has changed significantly since it is the sum of the raffinate flow and the flow of the water stream from the decanter, so F = 294.607 + 101.556 = 396.163 lbmol/h. The corresponding composition of the feed to the solvent recovery column is (0.0026, 0.0111, 0.9862). Here again we assume the column is a feed pinched stripper so the distillate composition, yD, from solvent stripper is in phase equilibrium with the feed composition to the solvent recovery column. This gives yD = (0.00391, 0.62852, 0.37108). Mass balance can be used to calculate the bottoms composition from the solvent recovery column, xB = (0.0021, 0.0033, 0.9946). This gives V = D = 4.977 lbmol/h, B = 395.978 lbmol/h and a boil-up ratio of s = 0.01256. Again, the energy demands for this solvent stripper remain insignificant.
Flowsheet Iteration #4: The primary variables are the solvent recycle flow and composition to the liquid-liquid extraction column, from which the make-up solvent flow can be calculated.
- Extraction Column. The initial estimate of the flowsheet
iteration variables are S1 = 445.401 lbmol/h and
xS1 = (0.00661,
0.82828, 0.16511). Again, the target extract composition is
xT =
(0.0890, 0.6204, 0.2906). Liquid-liquid extraction mass and phase
equilibrium calculations give new estimates of the extract flow,
raffinate flow rate and composition, and the solvent make-up
flow.
Table 8: Extract and Raffinate Stream Variables from Liquid-Liquid Extraction Column Stream Flow Rate (lbmol/h) Composition Extract 590.358 (0.0890, 0.6204, 0.2906) Raffinate 294.553 (0.00278, 0.01113, 0.98609) The solvent make-up flow rate has dropped to 0.62 lbmol/h. Note that the extract and raffinate flow rates have changed significantly but that the compositions of these streams changed very little. The additional digits are important to retain because they affect convergence of the flowsheet.
- Primary Acid Recovery Column. Returning to the primary acid
recovery column with the same bottoms composition specifications of
xB =
(0.9999, 0.00005, 0.00005) and minimum boil-up ratio of s = 10.92 gives
B = 49.527 lbmol/h and D = 540.831 lbmol/h. The distillate composition
remains fixed at yD = (0.0056, 0.6771, 0.3173).
- Decanter. Again, the feed to the decanter now
consists
of the overhead from both columns. As with the primary acid recovery
column, it is the flow rates of the product streams from the decanter
that change significantly. The compositions of the organic and water
phases, on the other hand, change very little. Table 9 gives the values
of the stream variables for the decanter.
Table 9: Decanter Stream Variables for Hybrid Separation of Acetic Acid/Water Stream Flow Rate (lbmol/h) Composition S1 445.162 (0.006484, 0.828577, 0.164939) S2 101.9873 (0.002638, 0.01109, 0.98626) - Solvent Recovery Column. Remember the flow rate and composition of the feed to the solvent recovery column are coupled to the decanter. Three iterations were required to converge the decanter and solvent recovery column variables. The values shown in Table 7 are the converged stream variables for the decanter. The converged variables for the solvent stripping column are B = 396.540 lbmol/h, xB = (0.00239, 0.00156, 0.99604), V = D = 6.55731 lbmol/h, yD = (0.02984, 0.578269, 0.400746). The values of V and B give a boil-up ratio of s = 0.01256. The sparged steam flow rate is S = V. Again, the energy demands for this solvent stripper remain insignificant.
Flowsheet Iteration #5: The primary variables are the solvent recycle flow and composition to the liquid-liquid extraction column, from which the make-up solvent flow can be calculated.
- Extraction Column. The estimate of the flowsheet
iteration variables are S1 = 445.162 lbmol/h and
xS1 = (0.006484,
0.828577, 0.164939). Again, the target extract composition is
xT =
(0.0890, 0.6204, 0.2906). Liquid-liquid extraction mass and phase
equilibrium calculations give new estimates of the extract flow,
raffinate flow rate and composition, and the solvent make-up flow. These
calculations give the following results:
Table 10: Extract and Raffinate Stream Variables from Liquid-Liquid Extraction Column Stream Flow Rate (lbmol/h) Composition Extract 589.864 (0.0890, 0.6204, 0.2906) Raffinate 294.563 (0.002731, 0.011116, 0.986152) The solvent make-up stream is 0.375 lbmol/h. Note that the extract and raffinate flow rates have changed significantly but that the compositions of these streams changed very little. However, the additional digits are important to retain because they affect convergence of the flowsheet.
- Primary Acid Recovery Column. Returning to the primary acid
recovery column with the same bottoms composition specifications of
xB =
(0.9999, 0.00005, 0.00005) and minimum boil-up ratio of s = 10.92 gives
B = 49.485 lbmol/h and D = 540.3788 lbmol/h. The distillate composition
remains fixed at yD = (0.0056, 0.6771, 0.3173).
- Decanter. Again, the feed to the decanter now consists
of the overhead from both columns. As with the primary acid recovery
column, it is the flow rates of the product streams from the decanter
that change significantly. The compositions of the organic and water
phases, on the other hand, change very little. Table 11 gives the values
of the stream variables for the decanter.
Table 11: Decanter Stream Variables for Hybrid Separation of Acetic Acid/Water Stream Flow Rate (lbmol/h) Composition S1 444.957 (0.006367, 0.828847, 0.164785) S2 103.7319 (0.002685, 0.011107, 0.986208 ) - Solvent Recovery Column. Remember the flow rate and composition of the feed to the solvent recovery column are coupled to the decanter. Three iterations were required to converge the decanter and solvent recovery column variables. The values shown in Table 9 are the converged stream variables for the decanter. The converged variables for the solvent stripping column are B = 396.7882 lbmol/h, xB = (0.00250, 0.000938, 0.99656), V = D = 8.20133 lbmol/h, yD = (0.011081, 0.49430, 0.49461). The values of V and B give a boil-up ratio of s = 0.01256. The sparged steam flow rate is S = 8.2858 lbmol/h.
Flowsheet Iteration #6: The primary variables are the solvent recycle flow and composition to the liquid-liquid extraction column, from which the make-up solvent flow can be calculated. At this point, all stream variables in the process flowsheet begin to change very little.
- Extraction Column. The estimate of the flowsheet iteration
variables are S1 = 444.957 lbmol/h and xS1 =
(0.006367, 0.828847,
0.164785). Again, the target extract composition is xT =
(0.0890,
0.6204, 0.2906). Liquid-liquid extraction mass and phase equilibrium
calculations give new estimates of the extract flow, raffinate flow rate
and composition, and the solvent make-up flow. These calculations give
the following results:
Table 12: Extract and Raffinate Stream Variables from Liquid-Liquid Extraction Column Stream Flow Rate (lbmol/h) Composition Extract 589.456 (0.0890, 0.6204, 0.2906) Raffinate 294.569 (0.00268772, 0.0111075, 0.986205) The solvent make-up stream is 0.175 lbmol/h. Note that the extract and raffinate flow rates and the compositions of these streams changed very little. However, the additional digits are important to retain because they affect convergence of the flowsheet.
- Primary Acid Recovery Column. Returning to the primary acid recovery column with the same bottoms composition specifications of xB = (0.9999, 0.00005, 0.00005) and minimum boil-up ratio of s = 10.92 gives B = 49.451 lbmol/h and D = 540.005 lbmol/h. The distillate composition remains fixed at yD = (0.0056, 0.6771, 0.3173).
- Decanter. At this stage of the computations, the flow
rates and compositions of the product streams from the decanter
change very little. Table 13 gives the values of the stream
variables for the decanter.
Table 13: Decanter Stream Variables for Hybrid Separation of Acetic Acid/Water Stream Flow Rate (lbmol/h) Composition S1 444.6828 (0.00638278, 0.828813, 0.164804) S2 103.9170 (0.00269150, 0.0111083, 0.986200) - Solvent Recovery Column. Remember the flow rate and composition of the feed to the solvent recovery column are coupled to the decanter. One iteration was required to converge the decanter and solvent recovery column variables. The values shown in Table 11 are the converged stream variables for the decanter. The converged variables for the solvent stripping column are B = 398.3009 lbmol/h, xB = (0.002564, 0.000433, 0.99700), V = D = 8.600868 lbmol/h, yD = (0.00568, 0.49431, 0.50000). The values of V and B give a boil-up ratio of s = 0.0216. The sparged steam flow rate is S = 8.600868 lbmol/h.
Flowsheet Iteration #7: This iteration is the next to last iteration for the process flowsheet.
- Extraction Column. The estimate of the flowsheet iteration
variables are S1 = 444.6828 lbmol/h and xS1 =
(0.00638278, 0.828813,
0.164804). Again, the target extract composition is xT =
(0.0890,
0.6204, 0.2906). Liquid-liquid extraction mass and phase equilibrium
calculations give new estimates of the extract flow, raffinate flow rate
and composition, and the solvent make-up flow. These calculations give
the following results:
Table 14: Extract and Raffinate Stream Variables from Liquid-Liquid Extraction Column Stream Flow Rate (lbmol/h) Composition Extract 589.469 (0.0890, 0.6204, 0.2906) Raffinate 294.523 (0.0026881, 0.0111078, 0.986203) The solvent make-up stream is 0.42 lbmol/h. Note that the extract and raffinate flow rates and the compositions of these streams changed very little. However, the additional digits are important to retain because they affect convergence of the flowsheet.
- Primary Acid Recovery Column. Returning to the primary acid recovery column with the same bottoms composition specifications of xB = (0.9999, 0.00005, 0.00005) and minimum boil-up ratio of s = 10.92 gives B = 49.452 lbmol/h and D = 540.017 lbmol/h. The distillate composition remains fixed at yD = (0.0056, 0.6771, 0.3173).
- Decanter. At this stage of the computations, the flow rates and
compositions of the product streams from the decanter change very
little. Table 15 gives the values of the stream variables for the
decanter.
Table 15: Decanter Stream Variables for Hybrid Separation of Acetic Acid/Water Stream Flow Rate (lbmol/h) Composition S1 444.69166 (0.00638489, 0.828808, 0.164807) S2 103.43418 (0.00269238, 0.0111085, 0.986199)) - Solvent Recovery Column. Remember the flow rate and composition of the feed to the solvent recovery column are coupled to the decanter. One iteration was required to converge the decanter and solvent recovery column variables. The values shown in Table 11 are the converged stream variables for the decanter. The converged variables for the solvent stripping column are B = 398.4405 lbmol/h, xB = (0.002573, 0.0010479, 0.996378), V = D = 8.108846 lbmol/h, yD = (0.0056859, 0.494314, 0.500000). The values of V and B give a boil-up ratio of s = 0.02035. The sparged steam flow rate is S = 8.108846 lbmol/h.
Flowsheet Iteration #8: This iteration is the last iteration for the process flowsheet calculations.
- Extraction Column. The estimate of the flowsheet iteration
variables are S1 = 444.69166 lbmol/h and xS1 =
(0.00638489, 0.828808,
0.164807). Again, the target extract composition is xT =
(0.0890,
0.6204, 0.2906). Liquid-liquid extraction mass and phase equilibrium
calculations give new estimates of the extract flow, raffinate flow rate
and composition, and the solvent make-up flow. These calculations give
the following results:
Table 16: Extract and Raffinate Stream Variables from Liquid-Liquid Extraction Column Stream Flow Rate (lbmol/h) Composition Extract 589.477 (0.0890, 0.6204, 0.2906) Raffinate 294.524 (0.0026896, 0.0111079, 0.986202) The solvent make-up stream remains at 0.42 lbmol/h.
- Primary Acid Recovery Column. Returning to the primary acid recovery column with the same bottoms composition specifications of xB = (0.9999, 0.00005, 0.00005) and minimum boil-up ratio of s = 10.92 gives B = 49.4527 lbmol/h and D = 540.024 lbmol/h. The distillate composition remains fixed at yD = (0.0056, 0.6771, 0.3173).
- Decanter. At this stage of the computations, the flow rates
and
compositions of the product streams from the decanter change very
little. Table 17 gives the values of the stream variables for the
decanter.
Table 17: Decanter Stream Variables for Hybrid Separation of Acetic Acid/Water Stream Flow Rate (lbmol/h) Composition S1 444.6907 (0.00638433, 0.828809, 0.164806) S2 103.43143 (0.00269215, 0.0111085, 0.986199) - Solvent Recovery Column. Remember, both the flow rate and composition of the feed stream to the solvent recovery column are coupled to the decanter. Only a single iteration was required to converge the decanter and solvent recovery column variables. The values shown in Table 11 are the converged stream variables for the decanter. The converged variables for the solvent stripping column are B = 397.9581 lbmol/h, xB = (0.0025746, 0.00104917, 0.996376), V = D = 8.098115 lbmol/h, yD = (0.0056857, 0.494314, 0.499999). The values of V and B give a boil-up ratio of s = 0.02035. The sparged steam flow rate is S = 8.098115 lbmol/h.
Comparing iterations 7 and 8, notice that all variables change very, very little so we conclude that the process flowsheet is converged. Figure 7.2 gives the converged flowsheet.
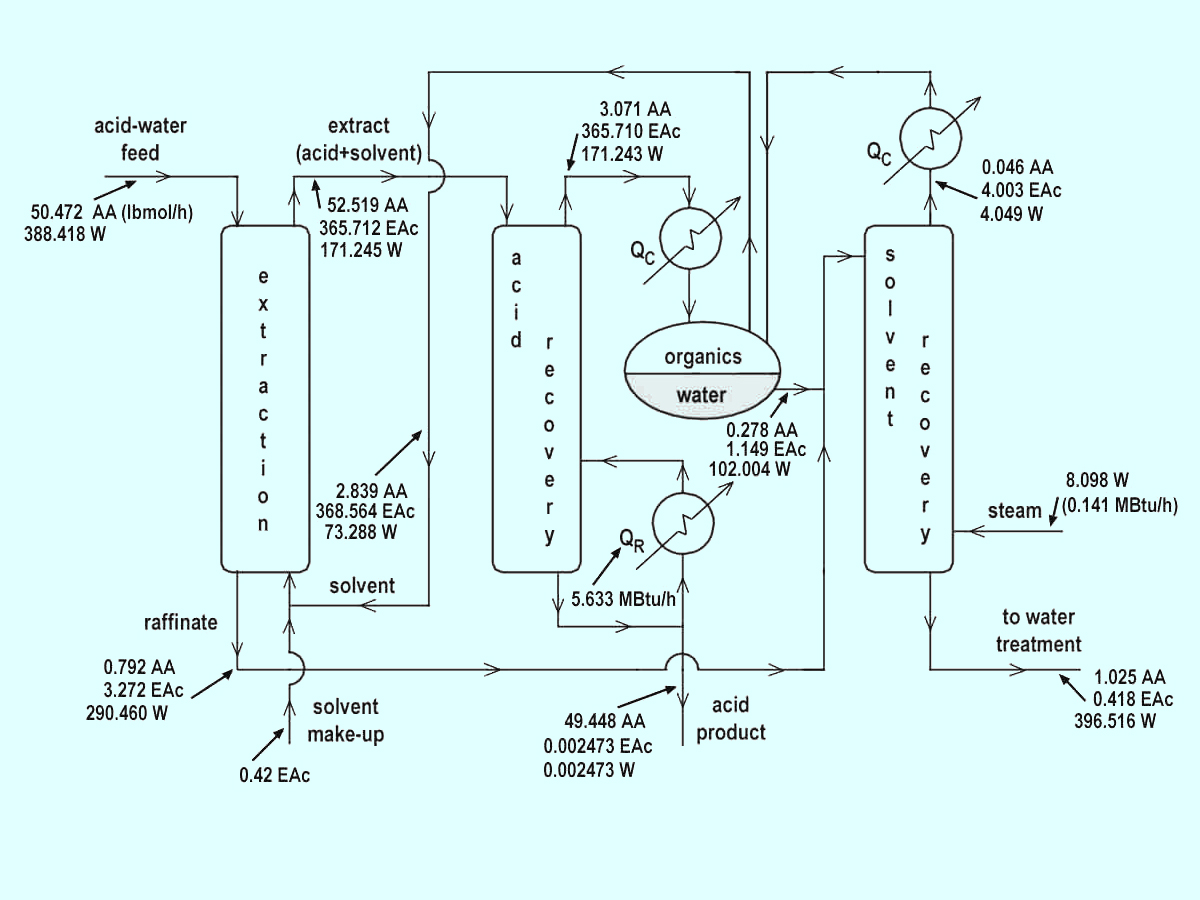
Figure 7.2: Hybrid Separation Process for Acid Production with Solvent Recycle
Energy Considerations in Extraction-Distillation Processes. We are now in a position to calculate the energy demands for the extraction-distillation process flowsheet. Using the boil-up ratios, bottoms stream flow rates from the primary acid and solvent recovery columns, and the latent heats of vaporization for the bottoms products from both strippers, we can calculate the total energy input requirements for the process. Energy demands for each column are approximated by the simple equation QR = sBλ, where λ is the heat of vaporization of the bottoms product.
Primary Acid Recovery Column
QR = sBλAA = (10.92)(49.4527
lbmol/h)(10,431.6 Btu/lbmol) = 5.633308 MBtu/h
Solvent Recovery Column
QR = sBλW = (0.02035)(397.9581
lbmol/h)(17,465.22 Btu/lbmol) = 0.141441 MBtu/h
Total Energy Demands for Extraction-Distillation Process
Qtotal = 5.633308 + 0.141441 = 5.774749 MBtu/h
Notice the following:
- The total energy demands for the extraction-distillation process are dominated by the acetic acid recovery column.
- The energy requirements for the extraction-distillation process
are just a little more than half of the energy demands for the straight
distillation of the same dilute solution of acetic acid.
- QR (straight distillation) = 10.957 MBtu/h
- Qtotal (extraction-distillation) = 5.775 MBtu/h
Exercises.
- Validate any complete flowsheet iteration for the extraction-distillation process. Be sure to calculate all flow rates and compositions for the liquid-liquid extraction column, the primary acid recovery column, the decanter, and the solvent recovery column using inner iteration if necessary to converge the decanter-solvent recovery column loop.
- Re-do the extraction-distillation process calculations using a solvent recovery column with a reboiler instead of sparged steam.
- Re-calculate the energy requirements for the extraction-distillation process with a solvent recovery column with a reboiler. Are there any significant differences? If so, what are the significant differences?
Acknowledgements. I would like to thank the National Science Foundation for support of this work under Grant No. CTS-0624889. I would also like to thank Professor Ross Taylor of Clarkson University for performing the liquid-liquid extraction calculations.